what types of elements form covalent bonds
The pair of electrons participating in this type of bonding is called shared pair or bonding pair. This type of covalent bond is formed when two atoms share an equal number of electrons.
 |
| How To Predict Number Of Bonds Each Element Forms Chemsimplified |
When none of the elements in a compound is a metal no atoms in the compound have an ionization energy low enough for electron loss to be likely.
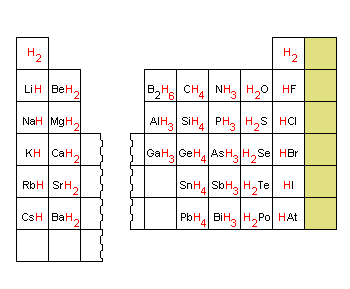
. Answer 1 of 25. The covalent bonding can be explained by using lewis dot structure concept. Covalent bonds form when atoms share valence electrons with other atoms to. The simplified version is that bonds between two non-metals are covalent whereas a bond between a non-metal and a metal is ionic though there are many exceptions.
The purest form of a covalent bond exists in diatomic gases. Single Covalent Bonds Between Different Atoms. The structure has two chlorine atoms in the structure which form covalent bond. A specific type of covalent bond.
These elements combine together to. A covalent bond is formed by equal sharing of electrons from both the participating atoms. When atoms of different. Covalent bonds form between what types of elements.
Unlike other types of bonding covalent bonding can exist between two atoms of the same elements resulting in the molecular form of the element. The simplest covalent bond exists in the diatomic. Some atoms can form more than one type of bond. 31 Covalent Bonding Worksheet Answers Chemistry.
Tamang sagot sa tanong. Hydrogen oxygen nitrogen and the halogens all form these types of bonds. In such a case. Only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms.
If one atom is on the far left Group 1 or 2. Based on the bond length covalent bonds are of the following types. By sharing an electron they satisfy. What are the 4 common elements that form covalent bonds.
Study with Quizlet and memorize flashcards containing terms like What types of elements form covalent bonds what does the line represent in a covalent bond what do the 2 dots above an. In such a case covalence. What type of covalent bonds form between elements with an electronegativity range of 05 to 20. Covalent bonds form between non metallic elements having similar or nearly similar electro negativities.
The electrons involved are in the outer shells of the atoms. Other elements that can form covalent bonds include nitrogen carbon and fluorine. This was suggested by GN. Elements with a difference in electronegativity in that range form a polar covalent.
If one of the. A covalent bond is a type of chemical bond characterized by the joint sharing of electron pairs between atoms. A covalent bond forms when two non-metal atoms share a pair of electrons. Therefore atoms which make covalent bonds can not be either give electrons or accept.
An atom that shares one or more of. For example metals such as magnesium. The difference in electronegativity between two atoms is zero. Bonding worksheet covalent chapter.
One way to predict whether a bond is ionic or covalent is to look how far apart the two atoms forming the bonds are in the periodic table. Covalent bond involves the sharing of electrons between atoms. When none of the elements in a compound is a metal no atoms in the compound have an ionization energy low enough for electron loss to be likely.
 |
| Covalent Bonds And Full Outer Shells Mooramo |
 |
| Chemical Bonds |
 |
| Covalent Bond Biology Dictionary |
 |
| How To Predict Number Of Bonds Each Element Forms Chemsimplified |
 |
| The Chemistry Of Hydrogen |
Posting Komentar untuk "what types of elements form covalent bonds"